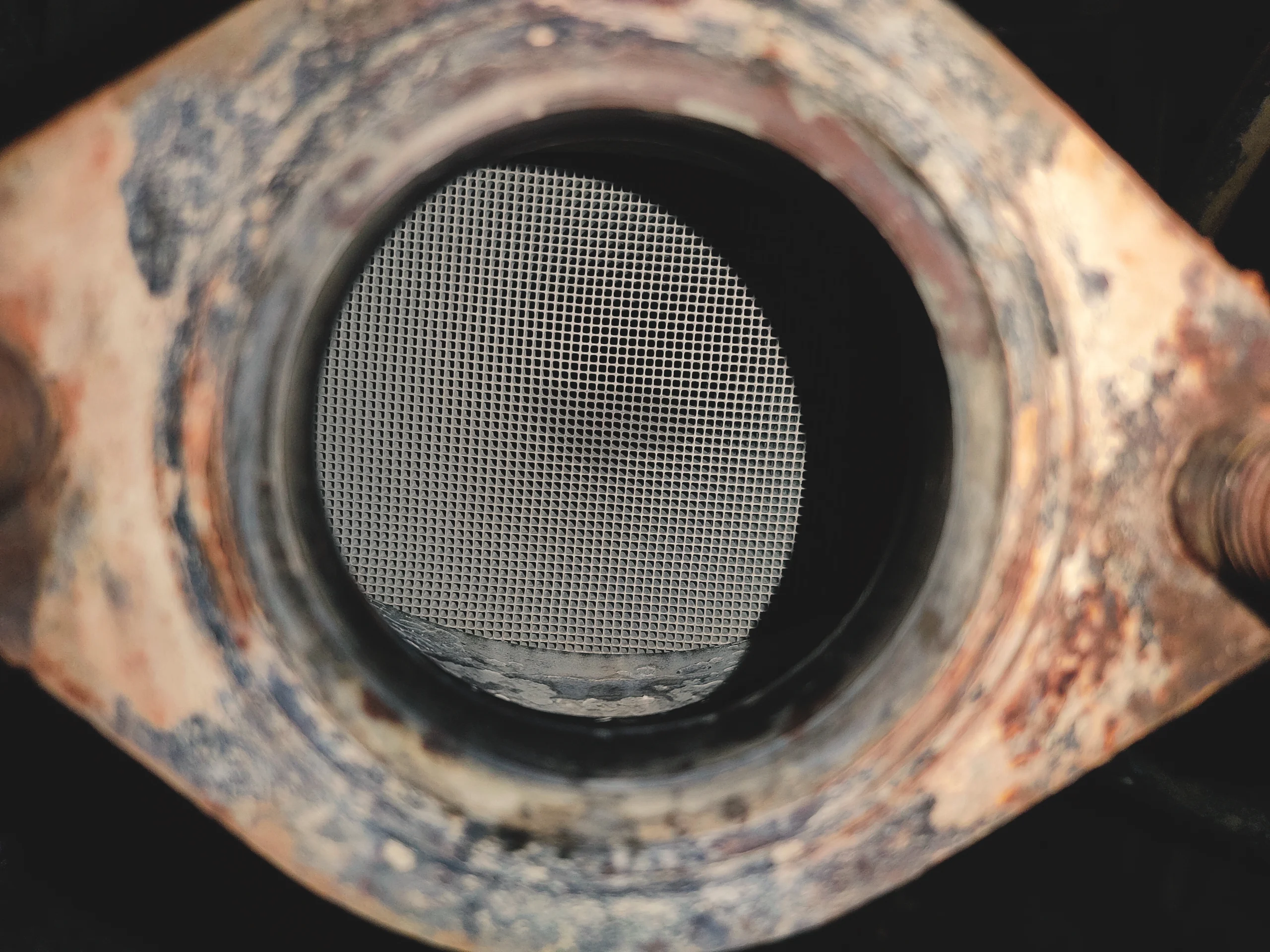
Catalytic converters are essential components in vehicles, specifically designed to minimize harmful emissions by converting toxic exhaust gases into less harmful substances. They are typically mounted as a critical part of the car’s exhaust system and utilize precious metals, which are responsible for their significant value.
Catalytic converters were introduced in the 1970s to combat growing air pollution concerns.
What Makes Catalytic Converters So Valuable?
The primary reason catalytic converters are so valuable is due to the presence of precious metals such as platinum, palladium, and rhodium—collectively known as platinum group metals (PGMs). These metals are critical catalysts used in a catalytic converter to facilitate the chemical reactions that transform harmful pollutants such as carbon monoxide, hydrocarbons, and nitrogen oxides into carbon dioxide, water, and nitrogen—significantly reducing environmental pollution.
Precious Metals Used in Catalytic Converters
Platinum (Pt)
Platinum is among the most commonly used precious metals in catalytic converters. Renowned for its resilience at high temperatures and exceptional catalytic properties, platinum efficiently converts harmful substances found in exhaust gases, such as carbon monoxide and hydrocarbons, into less harmful emissions. Typically, each converter contains around 1-2 grams of platinum.
Palladium (Pd)

Palladium is particularly prevalent in converters designed for gasoline vehicles. While generally less expensive than platinum, its catalytic efficiency and ability to resist corrosion at high temperatures make palladium highly valuable. Each catalytic converter can contain about 1-2 grams of palladium, varying depending on several factors, including the vehicle type and model.
Rhodium (Rh)
Rhodium, though used in smaller quantities due to its exceptionally high cost, is the most valuable precious metal found in catalytic converters. Its primary function is to significantly reduce nitrogen oxides, harmful emissions that gasoline and diesel engines emit during combustion. Rhodium usage typically varies from converter to converter but remains essential due to stringent emissions regulations.
Rhodium reached record-high prices in recent years, far surpassing gold and platinum, making catalytic converters even more attractive to thieves.
Why Do Thieves Target Catalytic Converters?
The rising prices of precious metals, especially rhodium, palladium, and platinum, have created a high demand for catalytic converters. Thieves often target these parts due to their valuable metal content, which can easily be sold as scrap metal. Thus, automobile catalytic converters have become a common target for theft, impacting vehicle owners globally.
Identifying the Value of Your Catalytic Converter
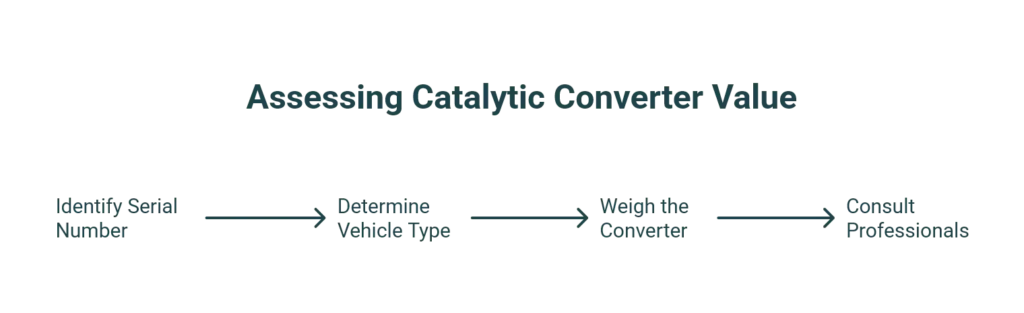
Determining the exact value of your catalytic converter involves understanding the type and amount of precious metals they contain. Here’s how you can assess the value:
- Check the Serial Number: Most catalytic converters feature a serial number, which helps identify their precise metal composition and corresponding value.
- Vehicle Type: Luxury and high-performance vehicles typically possess converters with higher amounts of precious metals. Diesel engines often contain different ratios, with higher concentrations of palladium and rhodium.
- Weigh the Converter: Generally, heavier converters indicate a higher quantity of valuable metals, though this method is less precise.
- Consult Professionals: The most accurate valuation is achieved by consulting professional catalytic converter recyclers like Recohub, who refine and recycle converters efficiently and responsibly.
Due to tightening of emission regulations globally, catalytic converters continue to increase in value, influencing the PGM market substantially.
Recycling and Refining Catalytic Converters with Recohub
Recohub specializes in recycling catalytic converters, playing a significant role in recovering valuable metals, reducing the need to mine new resources, and minimizing environmental damage. Refined recycled catalytic converters allow the reuse of precious metals they contain, thus significantly benefiting the environment and economy. Recycling with Recohub also helps mitigate the illegal trade in stolen converters, supporting a sustainable market.
Why Choose Recohub for Recycling?
Choosing Recohub ensures precious metal content is accurately assessed and refined. Recohub uses sophisticated methods to extract and refine valuable metals efficiently and responsibly, ensuring the highest return for your catalytic converter scrap while promoting sustainability.
In conclusion, the high value of catalytic converters primarily stems from their precious metal content, making them attractive for recycling, refining, and, unfortunately, theft. Understanding the metals they contain and their recycling potential allows vehicle owners to make informed decisions, ensuring maximum returns and supporting sustainable environmental practices.
FAQ
The use of precious metals in catalytic converters is primarily due to their exceptional ability to act as a catalyst in chemical reactions. Platinum, palladium, and rhodium are particularly effective at facilitating the conversion of harmful exhaust emissions into less harmful substances like carbon dioxide and water. These metals can withstand high temperatures and the corrosive environment within the exhaust system, making them indispensable in the process of reducing pollutants.
The value of catalytic converters stems from the precious metals they contain. With platinum group metals (PGMs) like platinum, palladium, and rhodium being rare and costly, the amount of precious metal in these devices makes them a prime target for scrap and recycle markets. These valuable metals are in high demand due to their effectiveness in reducing emissions and their limited natural supply.
Precious metals such as platinum are used in a catalytic converter as a catalyst to initiate and accelerate reactions that transform pollutants like carbon monoxide and hydrocarbons into less harmful substances. These metals are coated onto a ceramic substrate in the converter, creating a large surface area that facilitates interaction with exhaust gases.
The metals are responsible for converting toxic exhaust gases—such as carbon monoxide, nitrogen oxides, and unburned hydrocarbons—into less harmful substances like carbon dioxide, nitrogen, and water vapor, thereby ensuring the catalytic converter meets emissions standards and minimizes environmental impact.